
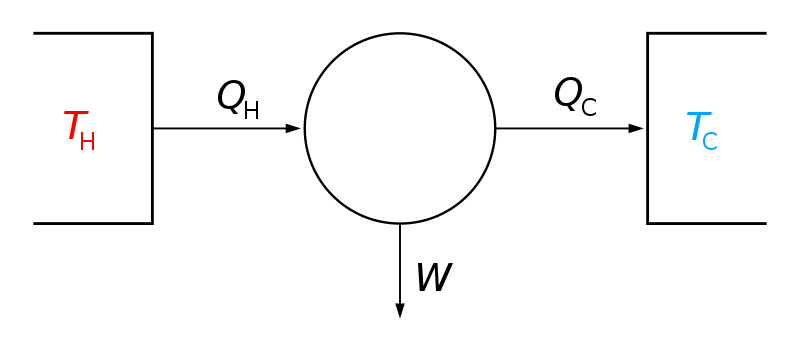

The second law of thermodynamics introduces a new thermodynamic property, entropy, and provides a mathematical statement that describes this unidirectional nature of processes. Processes have a natural direction to them in that spontaneous processes tend to dissipate gradients in the system until equilibrium is reached, e.g.:Ī system that is not subject to forced flows of mass or energy from its surroundings will evolve to a time-invariant state that is uniform or composed of uniform subsystems-the equilibrium state. Processes that satisfy these conservation equations may not be physically possible that is, the process of a cold cup of coffee spontaneously heating up on your dinner table would satisfy the first law of thermodynamics but has a near zero probability to occur. Conservation of total mass and energy are insufficient to solve many phase-equilibrium problems.


 0 kommentar(er)
0 kommentar(er)
